LABORATORY METHODS – PRACTICAL ASSIGNEMENT
INTERACTION BETWEEN GYPSUM AND A P-BEARING AQUEOUS SOLUTION
INSTRUCTIONS: Please follow the guidelines described in the tasks. Your assignmentshould be delivered in hardcopy, including your name, student ID number and the answers clearly listed by task. Failure to comply with communicated deadlines will involve a -10% per delayed day penalty on assignment mark.
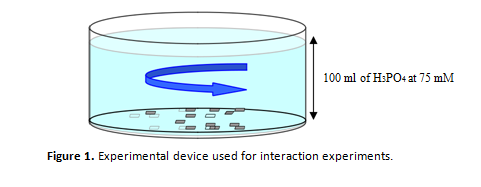
Experimental device
The followed experimental procedures consisted on the immersion of fragments of exfoliation of natural gypsum in 100 ml of a 75 mM phosphoric acid (H3PO4) aqueous solution, previously neutralized with sodium hydroxide (NaOH) until the desired initial pH value of 6 was attained. The experiments took place inside polypropylene batches, closed with Parafilm film in order to avoid dissolution evaporation, and were maintained at constant temperature of 25 ± 0.1 ºC and 1 atm of pressure. The dissolutions remained at constant 25 rpm stirring during the experiments, using floating stirrers covered with Teflon. Figure 1 shows a schematic representation of the used experimental device.
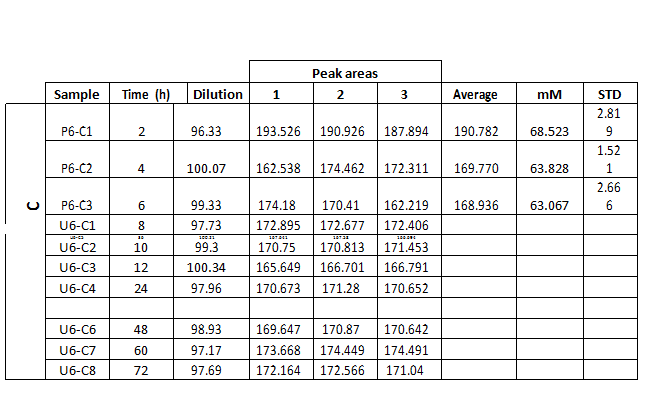
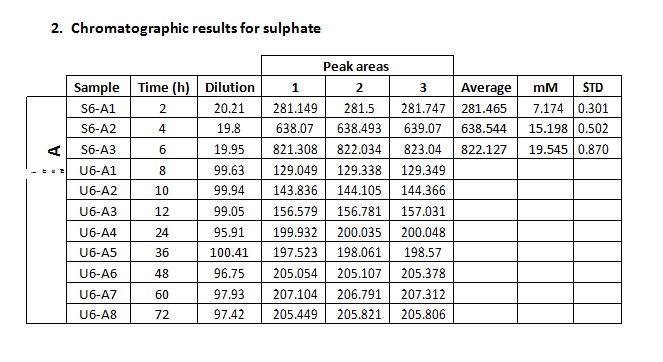
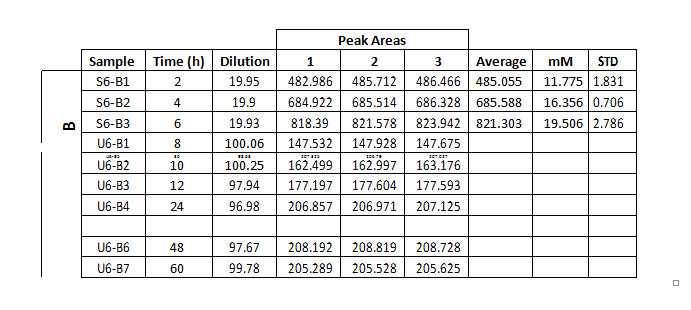
These experiments were designed to study the evolution of the aqueous solution chemistry during the interaction process. In this case, the grain sizes were selected to have diameters ranging from 1.0 to 1.5 mm. The duration of these experiments was of 72 hours, and dissolution samples were collected at 2, 4, 6, 8, 10, 12, 24, 36, 48, 60, 72 hours of elapsed time after inserting the gypsum grains in the reaction batch. Finally, each experiment was repeated three times in order to examine the reproducibility of the process.
I – ANION CONCENTRATIONS
IA. Analytical methods
The measure of total phosphate and sulphate concentrations in the aqueous dissolution was carried out by means of ionic chromatography, using a Metrohm Advanced Compact 861 IC chromatographer. Samples collected during the experimental stage
were diluted and compared with six reference dissolutions with different concentrations. These dissolutions were prepared using standard dissolutions of Na2SO4 and KHPO4, both having 1000 ± 2 ppm (Panreac) concentration.
An anion column (6.1006.520 Metrostep A SUPP 5 – 150) was used and the applied eluent was a dissolution of 15 mM of NaOH and 2.0 mM Na2CO3, with a flux velocity of 1.0 ml/min. and 5 MPa of pressure. The suppressor consisted of a solution with 100 mM of H2SO4.
IB. Calibration lines
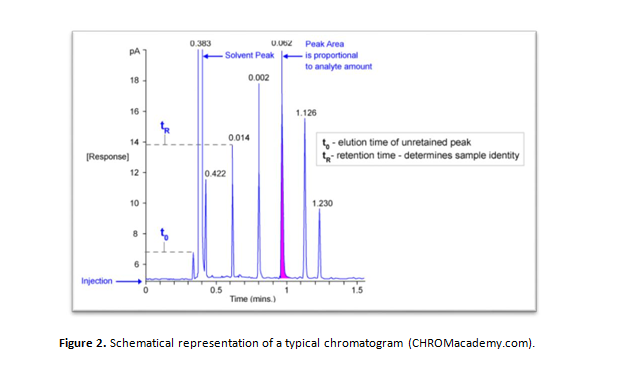
In ion chromatography, aqueous concentrations are measured in a chromatogram of retention time versus detector response (Figure 2). In this graphical representation, the retention time enables the identification of the component in question, while its concentration is proportional to the corresponding peak’s area. For more information on ion chromatography, please consult Harvey, 2000.
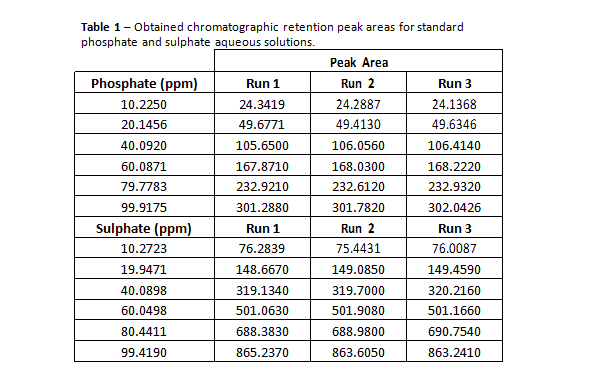
In the present case, we intend to measure sulphate and phosphate concentrations, and the results obtained from standard solutions are presented in table 1.
II – CALCIUM CONCENTRATIONS
IIA. Analytical methods
The concentrations of total calcium in dissolution were measured with a PYE-UNICAM SP9 atomic absorption/emission spectrophotometer equipped with an acetylene burner. The analyses were carried out using a calcium light bulb (422.7 nm of wave length).
Each sample was analysed eight times and the detection limit for calcium is 2 ppm. The reference dissolutions applied for all experiments were prepared using a standard dissolution of 1000 ppm (Panreac).
IIB. Calibration lines
Atomic Absorption Spectrometry (AAS) is a method requiring frequent re-calibration during operation procedures and for the sake of simplicity you will not be asked to perform the tedious task of finding a new calibration expression every 10 measurements. The final results for each of the 3 experiments are included in the Appendix: Data section.
If you need answers to this assignment, WhatsApp/Text +1 646 978 1313 or send us an email to admin@shrewdwriters.com and we will reply instantly. We provide original answers that are not plagiarized, please try our service.

Leave a Reply